what is the name given to the process when solvent molecules surround the solute molecules?

A sodium ion solvated by water molecules.
Solvation (or dissolution) describes the interaction of solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with solvent, and the strength and nature of this interaction influence many properties of the solute, including solubility, reactivity, and colour, also as influencing the properties of the solvent such as the viscosity and density.[1] In the process of solvation, ions are surrounded by a concentric trounce of solvent. Solvation is the process of reorganizing solvent and solute molecules into solvation complexes. Solvation involves bond germination, hydrogen bonding, and van der Waals forces. Solvation of a solute by water is called hydration.[2]
Solubility of solid compounds depends on a competition between lattice energy and solvation, including entropy furnishings related to changes in the solvent structure.[3]
Stardom from solubility [edit]
By an IUPAC definition,[four] solvation is an interaction of a solute with the solvent, which leads to stabilization of the solute species in the solution. In the solvated state, an ion in a solution is surrounded or complexed past solvent molecules. Solvated species can often be described by coordination number, and the complex stability constants. The concept of the solvation interaction can also be applied to an insoluble fabric, for case, solvation of functional groups on a surface of ion-commutation resin.
Solvation is, in concept, singled-out from solubility. Solvation or dissolution is a kinetic process and is quantified by its rate. Solubility quantifies the dynamic equilibrium state accomplished when the rate of dissolution equals the rate of atmospheric precipitation. The consideration of the units makes the stardom clearer. The typical unit for dissolution charge per unit is mol/s. The units for solubility express a concentration: mass per volume (mg/mL), molarity (mol/L), etc.[5]
Solvents and intermolecular interactions [edit]
Solvation involves different types of intermolecular interactions: hydrogen bonding, ion-dipole interactions, and van der Waals forces (which consist of dipole-dipole, dipole-induced dipole, and induced dipole-induced dipole interactions). Which of these forces are at play depends on the molecular structure and properties of the solvent and solute. The similarity or complementary character of these properties betwixt solvent and solute determines how well a solute tin can be solvated past a particular solvent.

Nile ruby-red at daylight (summit row) and UV-calorie-free (second row) in different solvents. From left to right: 1. Water, 2. Methanol, iii. Ethanol, iv. Acetonitrile, 5. Dimethylformamide, 6. Acetone, seven. Ethylacetate, 8. Dichlormethane 9. n-Hexane, x. Methyl-tert-Butylether, 11. Cyclohexane, 12. Toluene. Photographer: Armin Kübelbeck, CC-By-SA, Wikimedia Commons
Solvent polarity is the most important factor in determining how well information technology solvates a detail solute. Polar solvents have molecular dipoles, meaning that part of the solvent molecule has more electron density than another part of the molecule. The office with more electron density will feel a partial negative charge while the role with less electron density will feel a partial positive charge. Polar solvent molecules can solvate polar solutes and ions because they tin orient the appropriate partially charged portion of the molecule towards the solute through electrostatic attraction. This stabilizes the system and creates a solvation shell (or hydration shell in the case of water) around each particle of solute. The solvent molecules in the immediate vicinity of a solute particle oftentimes have a much different ordering than the rest of the solvent, and this area of differently ordered solvent molecules is called the cybotactic region.[6] Water is the most common and well-studied polar solvent, but others be, such equally ethanol, methanol, acetone, acetonitrile, and dimethyl sulfoxide. Polar solvents are oftentimes plant to accept a high dielectric abiding, although other solvent scales are as well used to classify solvent polarity. Polar solvents can be used to deliquesce inorganic or ionic compounds such every bit salts. The conductivity of a solution depends on the solvation of its ions. Nonpolar solvents cannot solvate ions, and ions volition be constitute as ion pairs.
Hydrogen bonding amongst solvent and solute molecules depends on the ability of each to accept H-bonds, donate H-bonds, or both. Solvents that can donate H-bonds are referred to as protic, while solvents that do not contain a polarized bail to a hydrogen atom and cannot donate a hydrogen bond are called aprotic. H-bond donor ability is classified on a scale (α).[vii] Protic solvents tin can solvate solutes that can accept hydrogen bonds. Similarly, solvents that can have a hydrogen bond tin solvate H-bond-altruistic solutes. The hydrogen bond acceptor ability of a solvent is classified on a scale (β).[eight] Solvents such equally water can both donate and take hydrogen bonds, making them first-class at solvating solutes that can donate or accept (or both) H-bonds.
Some chemical compounds experience solvatochromism, which is a alter in color due to solvent polarity. This phenomenon illustrates how dissimilar solvents interact differently with the same solute. Other solvent effects include conformational or isomeric preferences and changes in the acerbity of a solute.
Solvation energy and thermodynamic considerations [edit]
The solvation process will exist thermodynamically favored only if the overall Gibbs energy of the solution is decreased, compared to the Gibbs energy of the separated solvent and solid (or gas or liquid). This means that the change in enthalpy minus the change in entropy (multiplied by the absolute temperature) is a negative value, or that the Gibbs energy of the system decreases. A negative Gibbs energy indicates a spontaneous process but does non provide data about the rate of dissolution.
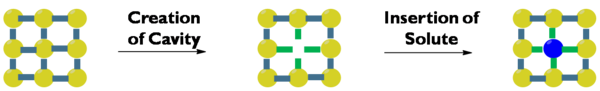
Solvation involves multiple steps with different energy consequences. First, a cavity must form in the solvent to make infinite for a solute. This is both entropically and enthalpically unfavorable, as solvent ordering increases and solvent-solvent interactions decrease. Stronger interactions among solvent molecules leads to a greater enthalpic penalty for cavity formation. Next, a particle of solute must separate from the majority. This is enthalpically unfavorable since solute-solute interactions decrease, but when the solute particle enters the cavity, the resulting solvent-solute interactions are enthalpically favorable. Finally, as solute mixes into solvent, at that place is an entropy gain.[6]
The enthalpy of solution is the solution enthalpy minus the enthalpy of the separate systems, whereas the entropy of solution is the corresponding difference in entropy. The solvation energy (change in Gibbs free energy) is the change in enthalpy minus the production of temperature (in Kelvin) times the change in entropy. Gases have a negative entropy of solution, due to the decrease in gaseous volume as gas dissolves. Since their enthalpy of solution does not decrease besides much with temperature, and their entropy of solution is negative and does non vary appreciably with temperature, near gases are less soluble at college temperatures.
Enthalpy of solvation tin can help explain why solvation occurs with some ionic lattices but not with others. The difference in energy between that which is necessary to release an ion from its lattice and the free energy given off when information technology combines with a solvent molecule is called the enthalpy change of solution. A negative value for the enthalpy change of solution corresponds to an ion that is likely to deliquesce, whereas a high positive value means that solvation will not occur. It is possible that an ion will deliquesce fifty-fifty if it has a positive enthalpy value. The extra energy required comes from the increase in entropy that results when the ion dissolves. The introduction of entropy makes it harder to make up one's mind by calculation alone whether a substance will dissolve or not. A quantitative measure for solvation power of solvents is given by donor numbers.[9]
Although early thinking was that a higher ratio of a cation's ion charge to ionic radius, or the charge density, resulted in more solvation, this does non stand upwardly to scrutiny for ions like iron(III) or lanthanides and actinides, which are readily hydrolyzed to form insoluble (hydrous) oxides. Equally these are solids, it is apparent that they are non solvated.
Potent solvent-solute interactions make the process of solvation more favorable. I mode to compare how favorable the dissolution of a solute is in different solvents is to consider the free free energy of transfer. The free energy of transfer quantifies the free energy departure between dilute solutions of a solute in two different solvents. This value essentially allows for comparison of solvation energies without including solute-solute interactions.[6]
In general, thermodynamic analysis of solutions is done past modeling them as reactions. For example, if you lot add together sodium chloride to h2o, the salt will dissociate into the ions sodium(+aq) and chloride(-aq). The equilibrium constant for this dissociation can be predicted by the change in Gibbs energy of this reaction.
The Born equation is used to estimate Gibbs free free energy of solvation of a gaseous ion.
Recent simulation studies accept shown that the variation in solvation energy betwixt the ions and the surrounding water molecules underlies the mechanism of the Hofmeister series.[10] [i]
Macromolecules and assemblies [edit]
Solvation (specifically, hydration) is important for many biological structures and processes. For instance, solvation of ions and/or of charged macromolecules, similar DNA and proteins, in aqueous solutions influences the germination of heterogeneous assemblies, which may exist responsible for biological office.[11] Some other case, poly peptide folding occurs spontaneously, in function because of a favorable modify in the interactions betwixt the protein and the surrounding water molecules. Folded proteins are stabilized past 5-10 kcal/mol relative to the unfolded land due to a combination of solvation and the stronger intramolecular interactions in the folded protein construction, including hydrogen bonding.[12] Minimizing the number of hydrophobic side-bondage exposed to water by burying them in the center of a folded protein is a driving force related to solvation.
Solvation as well affects host–guest complexation. Many host molecules have a hydrophobic pore that readily encapsulates a hydrophobic invitee. These interactions can be used in applications such every bit drug commitment, such that a hydrophobic drug molecule can exist delivered in a biological system without needing to covalently change the drug in guild to solubilize it. Binding constants for host–invitee complexes depend on the polarity of the solvent.[13]
Hydration affects electronic and vibrational properties of biomolecules.[14] [fifteen]
Importance of solvation in computer simulations [edit]
Due to the importance of the effects of solvation on the structure of macromolecules, early figurer simulations which attempted to model their behaviors without including the furnishings of solvent (in vacuo) could yield poor results when compared with experimental data obtained in solution. Small molecules may also adopt more than compact conformations when fake in vacuo , this is due to favorable Vaan Der Waals interactions and intramolecular electrostatic interactions which would exist dampened in the presence of a solvent.
As computer power increased it became possible to try and contain the effects of solvation within a simulation and the simplest way to do this is to surround the molecule being simulated with a "skin" of solvent molecules, akin to simulating the molecule within a drib of solvent if the skin is sufficiently deep.[16]
See also [edit]
- Saturated solution
- Solubility equilibrium
- Solvent models
- Born equation
- Supersaturation
- H2o model
References [edit]
- ^ a b M. Adreev; J. de Pable; A. Chremos; J. F. Douglas (2018). "Influence of Ion Solvation on the Backdrop of Electrolyte Solutions". J. Phys. Chem. B. 122 (14): 4029–4034. doi:10.1021/acs.jpcb.8b00518. PMID 29611710.
- ^ Cambell, Neil (2006). Chemistry - California Edition. Boston, Massachusetts: Pearson Prentice Hall. p. 734. ISBN978-0-13-201304-8.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 823. ISBN978-0-08-037941-8.
- ^ IUPAC, Compendium of Chemical Terminology, second ed. (the "Gold Book") (1997). Online corrected version: (2006–) "solvation". doi:10.1351/goldbook.S05747
- ^ Solubility – Common Measuring Units
- ^ a b c Eric V. Anslyn; Dennis A. Dougherty (2006). Mod Concrete Organic Chemistry. University Scientific discipline Books. ISBN 978-i-891389-31-3.
- ^ Taft R. West., Kamlet M. J. (1976). "The solvatochromic comparing method. 2. The .alpha.-scale of solvent hydrogen-bond donor (HBD) acidities". J. Am. Chem. Soc. 98 (10): 2886–2894. doi:10.1021/ja00426a036.
- ^ Taft R. W., Kamlet Thousand. J. (1976). "The solvatochromic comparison method. i. The .beta.-calibration of solvent hydrogen-bond acceptor (HBA) basicities". J. Am. Chem. Soc. 98 (ii): 377–383. doi:10.1021/ja00418a009.
- ^ Gutmann Five (1976). "Solvent furnishings on the reactivities of organometallic compounds". Coord. Chem. Rev. xviii (2): 225. doi:ten.1016/S0010-8545(00)82045-vii.
- ^ G. Adreev; A. Chremos; J. de Pablo; J. F. Douglas (2017). "Coarse-Grained Model of the Dynamics of Electrolyte Solutions". J. Phys. Chem. B. 121 (34): 8195–8202. doi:10.1021/acs.jpcb.7b04297. PMID 28816050.
- ^ A. Chremos; J. F. Douglas (2018). "Polyelectrolyte clan and solvation". The Journal of Chemical Physics. 149 (16): 163305. Bibcode:2018JChPh.149p3305C. doi:10.1063/1.5030530. PMC6217855. PMID 30384680.
- ^ Pace CN, Shirley BA, McNutt M, Gajiwala M (1996). "Forces contributing to the conformational stability of proteins". FASEB Journal. 10 (i): 75–83. doi:10.1096/fasebj.10.one.8566551. PMID 8566551. S2CID 20021399.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Steed, J. Due west. and Atwood, J. L. (2013) Supramolecular Chemistry. 2nd ed. Wiley. ISBN 1118681509, 9781118681503.
- ^ Mashaghi Alireza; et al. (2012). "Hydration strongly affects the molecular and electronic structure of membrane phospholipids". J. Chem. Phys. 136 (eleven): 114709. Bibcode:2012JChPh.136k4709M. doi:ten.1063/1.3694280. PMID 22443792.
- ^ Bonn Mischa; et al. (2012). "Interfacial H2o Facilitates Energy Transfer past Inducing Extended Vibrations in Membrane Lipids". J Phys Chem. 116 (22): 6455–6460. doi:10.1021/jp302478a. PMID 22594454.
- ^ Leach, Andrew R. (2001). Molecular modelling : principles and applications (2d ed.). Harlow, England: Prentice Hall. p. 320. ISBN0-582-38210-6. OCLC 45008511.
Further reading [edit]
- Dogonadze, Revaz; et al., eds. (1985–88). The Chemic Physics of Solvation (3 vols. ed.). Amsterdam: Elsevier. ISBN 0-444-42551-9 (function A), ISBN 0-444-42674-four (role B), ISBN 0-444-42984-0 (Chemistry)
- Jiang D., Urakawa A., Yulikov Thou., Mallat T., Jeschke G., Baiker A. (2009). "Size selectivity of a copper metal-organic framework and origin of catalytic activity in epoxide alcoholysis". Chemistry. 15 (45): 12255–62. doi:10.1002/chem.200901510. PMID 19806616.
{{cite periodical}}: CS1 maint: multiple names: authors list (link) [One case of a solvated MOF, where fractional dissolution is described.]
External links [edit]
| | Wikimedia Commons has media related to Solvation. |
| | Look up solvation in Wiktionary, the free dictionary. |
- Serafin, J.M. Transfer Gratuitous Free energy and the Hydrophobic Effect. J. Chem. Educ. 2003, lxxx, 1194-1196. http://pubs.acs.org/doi/pdf/10.1021/ed080p1194
Source: https://en.wikipedia.org/wiki/Solvation
0 Response to "what is the name given to the process when solvent molecules surround the solute molecules?"
Postar um comentário